 |
Welcome To Evlithium Best Store For Lithium Iron Phosphate (LiFePO4) Battery |
 |

NMC lithium-ion batteries—composed of nickel, manganese, and cobalt—are widely recognized for their high energy density and reliability, making them a preferred choice for various applications. They play a significant role in powering electric vehicles (EVs), portable electronics, energy storage systems, and more. Understanding their structure, functionality, and comparisons with other battery types can help you choose the right battery for your needs.
NMC batteries combine the advantages of nickel (high specific energy), manganese (thermal stability), and cobalt (reduced cathode corrosion). Their ability to store large energy in a small mass makes them highly efficient. However, challenges like cobalt’s environmental and ethical concerns drive manufacturers to minimize its use.
Charging: Lithium ions flow from the cathode to the anode via the electrolyte, while electrons travel through an external circuit.
Discharging: The process reverses, with ions moving back to the cathode and energy released to power devices.
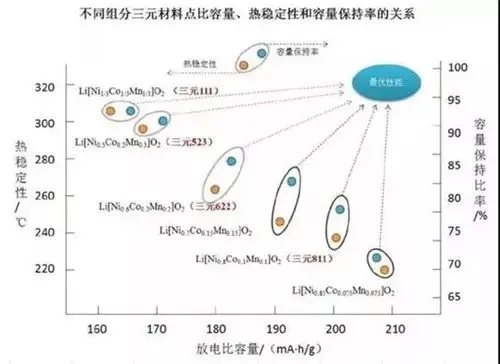
NMC 111: Equal parts nickel, manganese, and cobalt; balanced energy density and affordability.
NMC 532: Higher nickel content for increased energy density.
NMC 622: More cobalt, offering better thermal stability.
NMC 811: Dominated by nickel, minimizing cobalt usage while maximizing energy density.
| Feature | NMC Batteries | LFP Batteries |
|---|---|---|
| Specific Energy | Higher; compact energy storage. | Lower; requires larger volume. |
| Cycle Life | ~800 cycles. | ~3000+ cycles; longer lifespan. |
| Thermal Stability | Less stable, higher overheating risk. | Highly stable, lower overheating risk. |
| Cost | More expensive. | More affordable. |
| Environmental Impact | Less eco-friendly (toxic cobalt). | Environmentally friendly (non-toxic materials). |
| Applications | EVs, smartphones, laptops. | Electric buses, grid storage, stationary systems. |
NMC lithium-ion batteries are essential for industries requiring compact, high-energy storage solutions. Despite their advantages, considerations like cost, lifespan, and environmental impact are crucial when choosing between NMC and other alternatives, such as LFP batteries. The ongoing shift toward reducing cobalt in NMC batteries reflects the industry's drive for sustainability and efficiency.
Edit by paco
All Rights reserved © 2025 Evlithium Limited